DIY HHO Hydrogen Production a Water Fuel Cell
![]() DANGER: This project involves creating a mixture of Hydrogen and Oxygen which is a highly EXPLOSIVE GAS. When contained in a confined space, detonation of the gas would be highly dangerous and could cause serious injury.
DANGER: This project involves creating a mixture of Hydrogen and Oxygen which is a highly EXPLOSIVE GAS. When contained in a confined space, detonation of the gas would be highly dangerous and could cause serious injury.
 How it works
How it works
Water is a compound made from the two elements of Hydrogen and Oxygen. It has the chemical symbol H2O which indicates that each molecule is a combination of one Oxygen atom and two Hydrogen atoms.
All atoms can form ‘ions’. These are just the same atom except with a little extra charge. Atoms can become ionized when in the presence an electric field. You can see extreme examples of this in the DIY Tesla Coil project. Hydrogen forms positive ions, and oxygen forms negative ions. We use this to our advantage by using an electric field to pull the water molecules apart.
By placing two electrodes (metal plates) into water we can create an electric field between them by connecting them to the terminals of a battery or power supply. The positive electrode is known as the anode, while the negative one is the cathode. Pure water actually does not conduct electricity so it is not suitable to be used without adding something to the water. Tap water already contains many dissolved compounds which allow the water to conduct. The ions formed in the water will be attracted to the electrode of opposite polarity, i.e. the positive hydrogen ions will move towards the cathode, while the negative oxygen ions move to the anode. Once the ions reach the surface of the electrodes the charges will be neutralised by adding or removing electrons. The gas is then fee to bubble up out of the remaining water to be collected.
 The electrodes are typically made from metal or graphite (carbon) so that they can pass electricity into the water. It is important that the chosen material does not react readily with oxygen or one of the dissolved compounds otherwise reactions will occur at the surface of the cathode (negative electrode) and the water will become polluted with the products of the reactions. You will see an example of this below when copper electrodes are used. This also means that no or very little oxygen gas is released as it gets combined with the metal electrode and remains in the container.
The electrodes are typically made from metal or graphite (carbon) so that they can pass electricity into the water. It is important that the chosen material does not react readily with oxygen or one of the dissolved compounds otherwise reactions will occur at the surface of the cathode (negative electrode) and the water will become polluted with the products of the reactions. You will see an example of this below when copper electrodes are used. This also means that no or very little oxygen gas is released as it gets combined with the metal electrode and remains in the container.
 The Project
The Project
This is a simple project that is used to create Hydrogen and Oxygen gas by electrolysis of water. The aim was to get good gas production rates without using extra chemicals or eroding the electrodes.
The first electrodes tried were ones left over from a different project. They were made from Copper coated Carbon rods which are not ideal due to copper being able to react with the water. The idea was that the copper would eventually all react away and there would be just Carbon left which would not pollute the water.
The copper seemed to take too long to react away and it was decided that this would not be useful at all. Below you can see the result of using copper electrode for electrolysis. The blue sludge floating on the surface of the water is some reactant of the copper and tap water.
Many people use electrodes made from stainless steel kitchen ware or switch plates because the stainless steel does not react as easily. The problem is that the grade of the steel often found in such items is not great and you will be left with a brown sludge after a few minuets of operation. They are also quite thin, usually less than 1mm, which means that the do not last a very long time before being totally eroded away. The erosion of the electrodes happens much more quickly when high currents or solutes (often called catalysts) are used.
The volume of gas produced is proportional to the charge passing through the water (current) and therefore high current means more gas. To do this the spacing of the electrodes must be as close as possible while still having enough room for the gas to bubble out freely.
The metal chosen for the plates was special high grade stainless steel to reduce corrosion. Such metal is not as conductive as others like copper for instance, so these plates were made from thick sheets of 2mm to counter this potential limiting factor. Very high quality metal was used which meant it was too hard to cut with common DIY tools so these plates were cut using a high pressure water jet.
INFORMATION: Even the highest grade stainless steel will have some reaction with water and can produce toxic chemicals. Avoid touching the water after use.

The plates are layered on top of each other with nylon washers between used as spacing. They are placed in alternating positions so that the plates would be +-+-+-. Stainless steel fixings were then used to fit it all together. It is important that it is put together well otherwise sparks could occur in the gas production area resulting in an explosion.
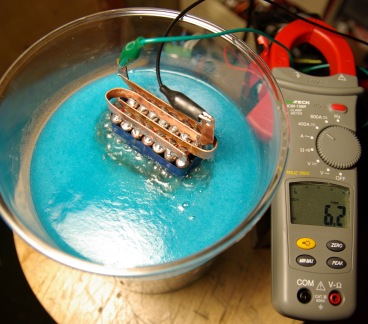
 A total of 16 plates were used in with 1mm spacing between each of them. The large combined surface area and thickness of the plates and bolts meant that this could carry very large currents without significant resistive heating in the metal. The total capacitance of the electrodes was 1nF when measured in air which indicates a large close surface area for gas production. This set of electrodes would draw about 25A from ordinary tap water. To collect the gas, the electrodes need to be placed in some sort of container. The container used was just something from a supermarket and was originally intended for storing something like tea!
A total of 16 plates were used in with 1mm spacing between each of them. The large combined surface area and thickness of the plates and bolts meant that this could carry very large currents without significant resistive heating in the metal. The total capacitance of the electrodes was 1nF when measured in air which indicates a large close surface area for gas production. This set of electrodes would draw about 25A from ordinary tap water. To collect the gas, the electrodes need to be placed in some sort of container. The container used was just something from a supermarket and was originally intended for storing something like tea!
This video shows the result of applying 12V to the electrodes when submerged in ordinary tap water. No ‘catalysts’ have been added to the water at all, this is just tap water!
It is drawing about 25A. Power to the cell is controlled using a pulse width modulation circuit.

 The container was made from metal so it was important to place the electrodes on a plastic base to prevent any short circuits. This image shows how two banana sockets were installed either side of some copper and brass fittings used to extract the gas. The power and pipe fittings were screwed tightly and sealed with silicon sealant so that the closed container would be air tight.
The container was made from metal so it was important to place the electrodes on a plastic base to prevent any short circuits. This image shows how two banana sockets were installed either side of some copper and brass fittings used to extract the gas. The power and pipe fittings were screwed tightly and sealed with silicon sealant so that the closed container would be air tight.
The gas produced is a highly explosive mixture of Hydrogen and Oxygen and should be treated with extreme caution. A large volume of gas exists inside the container which if ignited would explode and destroy the container. To avoid detonating the gas, the pipe from the container is fed into the base of another container which is half filled with water. This allows the gas to bubble trough the water to then be collected via another pipe which is used as the gas output. Now if any ignition occurs at the output, the flames can’t get back past the bubbler device and into the large gas volume in the electrolysis cell. This is an absolutely essential safety device and should not be skipped.
Now it is just deciding what to do with the gas! A good way to see the how explosive the gas mixture is to bubble the gas through another container of water such as a mug and ignite the bubbles as they reach the surface. Each bubble will explode very loudly and probably blow out the lighter.
A similar project which uses the explosive properties of the gas is the Hydrogen Cannon experiment.
![]() You should be aware that detonating this HHO gas mixture is VERY VERY loud.
You should be aware that detonating this HHO gas mixture is VERY VERY loud.









Hi
For added safety place “Bronze Wool” in connecting pipes and at the last output pipe.
Not much is needed, about 2 inch stuffed in will do it, this will act as a flash guard, it will stop the flame [if needed] from acting like a fuse being carried back to larger containers.
The larger containers make nice bombs….
BE CAREFUL
If running a long hose, Bronze Wool in the end of the hose !!!
Thank you for sharing this article, it’s really good. I Iearned many useful things from the article, I like the project that is used to create Hydrogen and Oxygen gas by electrolysis of water. The aim was to get good gas production rates without using extra chemicals or eroding the electrodes.
To be honest, when I am in high school, I can do some chemistry experiment. But now I forget all of them.
Hey again.
IT is possible to go overunity. If I understood correctly when using pulsed dc the wave reflects back on the off cycle and when reaching the on wave it creates and amplitude wave the sum of the 2 so if it was a 12v wave it becomes 24v…
And another idea I’m researching is when u use the pulsed dc in several pulses at the resonance freq of a toroidal coils u ad to your setup and that of water it reaches easily overunity or above faradays law.
By using Meyer’s way through restricted electron flow he could get his voltage to a point were it has the ability to go to infinity.
Another idea that Moray talks of is drawing energy from the water through cavitation where turbulence in the water can cause small bubbles to implode on themselves forming charged water particles greatly adding to the energy of the gas you produce with no additional voltage input.
Another trick is to disconnect the battery terminal for half an hour as to reset your ECU then after it starts again it will compensate for the extra O in the exhaust gasses. Voila! This I learned from a friend I made thats been building these setups for years and tested and installed something like 500 cars.
Hey there. Im so happy to have found your site. I have been researching HHO for the past year and a halve extensively and can provide some info aswell and would like to make some suggestions.
It is as I understand from Faraday’s Law better to have 1.6v – 2v per water spacing. So when using 12v its good to use 5 neutral plates in between your pos and neg then it splits the voltage per gap so 6 spacings = 2v.
Also I was thinking last night after reading on the induction heating thread that you used a coil and capacitor to resonate and that was what Meyer dit but his tube array was used as a capacitor and fashioned a coil right next to the tube in schematic and using pulsed step charging to resonate the coil.
Whats interesting is that he didnt use electrolysis to fracture the water. He used high voltage with RESTRICTED electron flow so as to make the molecule oscillate and reach an high energy state to the point of ionizing by itself known as gas ionizing stage. Produces huge amounts of gas with a few milliamps.
Also to consider is to treat the plates by just sanding for ex. 4 times upwards and 4 times sideways both sides and not touching the plates on the reactive surface.
It is not possible to make a car run on water. What people are doing is splitting water with electricity so that Hydrogen and Oxygen gas (HHO gas) can be fed into the air intake of their vehicle.
While the energy gained from burning this will be lower than the energy used to split the water, it may be possible to improve the efficinecy of the fuel combustion in the engine cylinder by adding this gas. The standard internal combustion engine is highly inefficient in terms of the conversion of chemical energy in fuel to kinetic energy in the engine so there is plenty of room for potentially improving this.
Dont forget that to split the water you are using electricity generated by the car altinator which therefore adds more resistance to the engine. You must also be careful not to pull too much current as it will burn out your altinator.
With modern engines the engine management system is likely to interfere with what you are trying to do as it changes the fuel air mix based on sensor readings like the lambda Oxygen sesor.
I have never tested it myself so I can not say if doing this will actually improve efficiency or not. We sell a range of quality PWM Control Circuits that could be used for producing HHO. We can also build custom ones if needed.
Ah greetings, so glad I am to locate your distinguished page. I have been looked on internets for nearly hour and now I weary from so all much looking.
I read all project and all comment but have not for yet locate exact circuit I wish. I request you show to me circuit, that make my car go with water(H2O) as I am weary of paying the gas to put in. Car should go fast, so please be making circuit deliver at least 100 amperes. How is to make water burn I don’t know. Is this reason for purpose of NaOH add to water? If you could provide such circuit quickly, I appreciate. Please be remembering that I am beginner so must be circuit simple to build with for not much tools.
And also very grateful i am be, if you make show to me machine of perpetuous moving, and also I am wanting magic guitar that knows millions of note so I look like mick jager when I play.
Thanks ;-D
(sorry, I couldn’t resist, after reading some of these comments lol! Great site, thanks for all the info and for all of your hard work here! You are clearly a cut above, with respect to your knowledge and customer service. I hope that your business is thriving; it certainly deserves to!
Best wishes 🙂
10cm x 10cm. LPM unknown.
I want to ask about the dimension of your cell and the produced hydrogen amount ( how many LPM ?) …. thanks
good morning
I want the device to apply in a hydrogen internal combustion engine
for use of a motorcycle
We had these made at a local metal processing shop. We may have some in the shop in future. Sign up to the newsletter to get product updates.
Hi I would like to know where I can get some stainless steel electrodes like the one’s in your diy hydrogen generator project. I would appreciate it. Thank You.

Yes, but the jet would need to be designed specifically to work with this fuel.
can the hydrogen from this plant can be used to power pulsejet engines ??
Yes, but you would need a different electrode configuration for the gasses to be separated.
Is it possible to separate the oxygen and hydrogen in quantities sufficient enough to say get 20liters of O2 a minute?
“5000volts and 0.8 amps.”
I do believe you would be even more impressed if changed your ratio to 1.6 volts and 2500 amps.
In order to produce an electrolytic reaction within the water it isn’t necessary to have such high voltages, technically I believe the bare minimum is 1.23V to initiate a reaction. In order to improve gas production, you would want to increase amps while maintaining the minimum voltage requirement for your given water/electrolyte solution.
Yep.
As a hydrogen cell is producing gas then the water level is depleting but it is only hydrogen and oxygen that that is being removed therefore all of the catelist still remains in the existing water. Therefore the strength of the catelist mixed with the water increases as the water level depletes until the water level is topped up. Therefore there is only need to add the catelist once because it is never removed from the water.
Do you agree with this theory?
DC current is necessary.
Which would provide better gas production, AC or DC current?
Depending on if you have the available means, gold coated electrodes would provide better conductivity than SS without adding sludge as gold does not oxidize.
The plates shown in the video are about 1mm apart.
Hello. I built a gas ” generator; HH O “. I used the plates of a transformer (4.5*3 cm) pluggin on the poles V+ L. The gaseous emission is very weak. (on your video it is important). Which must be the space between the plates? thank you.
I don’t think neutral plates help much. I never tried to get the cell to resonate as it was not necessary for the project.
thanks for your rapid response.
I`ve been using distilled water with sodium hydroxide as a catalist but wouldn`t it be better if you used the parameters of your choice to achieve maximum gas production for minimum power consumption?
I noticed that you don`t include any neutral plates in your cell. Is this because you have found that they don`t affect gas production?
Are you using the ppm to find resonating frequency for your cell?
thanks
I don’t know exactly but I can measure it for you if you provide a few more parameters.
The gas production is mostly related to the current flow but there are also other factors such as temperature, ion concentration, pressure etc.
You want me to test them with plain tap water? If so, is it at room temperature or something else?
Are you able to give me an idea of how much gas would be produced in a hydrogen cell using the 16 ss plates that you have available whilst pulling 25 amps?
Do you think that the efficiency of a hydrogen cell is judged by the power consumed and the quantity of gas produced?
My hydrogen cell produces 0.4 litres of gas/min whilst pulling 26 amps which is dismal, but I wouldn`t want to spend £100 opn a new set of plates just to produce the same amount of gas.
I have made the rms power pulse modultor hoping that a pulsed dc current would produce more gas but this didn`t happen although I was able to slow down the gas production using the ppm.
thanks
isn’t it true you can break down water into HHO gas by passing it through an
electric field, i don’t know how strong an electric field, maybe someone can comment on that. just getting some thoughts out there
MESSAGE FOR WILLY I am also interested by the production of gas starting from l’ water and of current…. There are 2 methods: that of MEYER which uses the resonance of l’ water with 43.430 kz! and that of NAUDIN my preference which uses an electric arc under a few kilo volts +20à40. In l’ water there is a little sodium bicarbonate….I think that you utlized a PWM …..thanks saying to me on gonin.jean@neuf.fr if your test with bicarbonate gives you good performances
I don’t know what effect it will have on your engine.
That website suggests sing tap water which is definitely not pure. Tap water will conduct easily. You can’t use pure water for electrolysis unless you used some sort of very high voltage system.
in my country, there’s a great HHO products that use only pure water but it said can be produced a very lot of HHO Gas.
i wonder if it’s true or just a bullsh**
thanks 😀
hi rm, is strong catalyst like NaOH hazardous especially for the engine ? all i knew is it still ok as long as it [the water] doesn’t sucked by the engine so only the steam/gass itself permitted to sucked up by the engine.. thank’s 😀
No that wont be pure water but I guess it would contain less dissolved ions than tap water.
Pure water wont work becasue it is a dielectric (insulator). The amount of gas produced is proportional to the flow of current.
A voltage multiplier wont work as it decreases the available current.
dear rm, is it possible to use only pure water without any electrolyte/catalyst as an enhancer to produce a lot of HHO Gas ?
i’ve tried without any catalyst but only a very little of bubble disperse from water itself.. even i’ve used it with PWM module [Homemade Power Pulse Controller]. i’m using 2 SS304 pipe.
can i multiply the voltage then use it with PWM [ combine the PWM with Inverter/Voltage Multiplier in your site ?] i’ven’t tried it yet.
thank’s i want to make HHO gas from only a pure water. 😀
thank’s
hi rm, whaddya mean by contamination ? is it like some ash in the water ? i’m using AC ex.water so it should be pure bcoz it’s from air condensation right ?
but i still use strong catalyst[NaOH] to enhance the HHO production. if i don’t use any catalyst then there’s was no HHO gas produced.
thank’s 🙂
Any contamination of the water could hasten the breakdown of your electrodes.
Even ss will still rust when there is large current.
hello Rm & all,
i’ve made my HHO kits using 2 SS304 Pipe.
and i connected it with ss plate as for the connector. bcoz before i’ve used only a copper wire then it became rusted so it can’t be used anymore.
but now the probs is it doesn’t connected again. when i tested with avometer it shows connected but with some resistant..
i’m using AC[airconditioner] ex. water without any catalyst.
i wonder why is my SS304 errode/covered with brown stain ?
thank’s 🙂
Yes you can parallel the MOSFETS. You should use at low frequency though for better efficiency.
I have just ordered an electrode kit(2 pair) from your company. I would like to power it from a 12V car battery and a simple PWM controler that I am building per your site. If I parallel the T1 (IRF740) will this work with the amperage involved? If not what would? Thanks
I generally like my gas to Explode,but not until i am ready. We are using Carbon rods from D-Cells, in a 15mm PVC pipe.
A plastic bag is held on to the pipe with a rubber band and removed when the bag is inflated. The gas can be drawn off into a syringe and injected into 35mm film cans. The plan is to take Photographs as they ignite.
I wont be injecting Hydrogen into my Transit, unless the Missus is driving.
Jon
Do you have a website or email that you could share for an example circuit?
I should imagine they would work. How well depends on the material it is made from. Most metals would react with the Oxygen causing the water to become sludgy quite quickly and the electrodes themselves to erode away. One possible advantage of this is that the Oxygen gets used up and therefore the gas you produce is just Hydrogen. The container full of hydrogen would not explode without any oxygen.
You could use an air spaced capacitor for the plates. Not an ideal solution but they do work.
Imagine this, using your Pulse width Modulator, connected to a neon sign transformer, which then gets connected into the cell. On the Neon sign transformer Im using the output is 5000volts and 0.8 amps. I think you will be amazed at your results. Although I am using graphite plates as my electrodes, as they are extremely conductive and seem to output almost twice the amount of gas as the stainless steel did for me. And with the current staying under 1 amp the water never heats up.